Due to the assortment of factors that can determine whether sterilization was successful or not, it is important that dentists regularly evaluate how efficient their in-office sterilizers are. The CDC and ADA recommends reviewing sterilizer operating procedures including packaging, loading, and spore testing, with all persons who work with the sterilizer to determine whether operator error could be a cause of a positive test.
Below are a few key recommendations from the ADA and CDC to keep in mind when determining your sterilizer monitoring procedure.
Sterilization Monitoring Best Practices
Sterilization is most effectively monitored when you use a combo of mechanical techniques, chemical indicators and biological indicators.Mechanical Monitoring
Mechanical monitoring is achieved by analyzing the temperature, cycle time and pressure gauges/displays on the sterilizer and recording the readouts for each cycle.Although accurate readings of these parameters does not necessarily ensure sterilization, an incorrect reading could be the first indicator of trouble with the cycle.
-
Chemical Indicators
Use a chemical indicator, such as internal or external indicator strips or tape, with each sterilization load. These types of indicators will assess the physical conditions of the sterilization process and change color when exposed to the correct sterilization environment (e.g., time and temperature). However, unless a Class 5 Integrating Indicator is used, chemical indicators DO NOT prove sterilization has been achieved.
-
Biological Indicators
According to the CDC, Biological Indicators are the most embraced method to monitor your sterilization process. These indicators don't simply test for the physical and chemical parameters for sterilization, they evaluate the process by actually killing highly resistant organisms like Bacillus or Stearothermophilus.
You should verify your sterilization cycle is functioning correctly by the periodic use (at least 1x a week) of Biological indicators. Although, when a load contains implantable devices it should be monitored with biological indicators and quarantined until results come in.
Types of Biological Monitoring
-
In-Office Biological Monitoring
It's an ideal way of testing especially when your office needs to test daily. For example, the CDC guidelines state every load that contains implantable devices should be monitored with biological indicators.
Although In-Office testing is a relatively new procedure to dental offices, its something that has been used in hospitals for quite some time. In-office biological monitoring systems typically include spore test strips, culture tubes, the proper incubator system and give results in 24-48 hours or 10 hours if using Confirm 10 In-Office Biological Monitoring system.
-
Mail-In Biological Monitoring
Mail-In monitoring services are one of the most cost-effective way to test sterilizers using biological indicators. When using this type of process it usually takes 1 week in order to receive sterilizer results. Although using mail-in monitoring systems take longer to receive results, when you use a third-party monitoring program it can offer more credibility to your records than in-house monitoring.
Some dental health-care professionals have concerns that delaying the results by having to mail specimens can cause false-negatives, but studies have determined mail delays have no effect on test results.
Not sure on the regulations for your state? Use the chart below
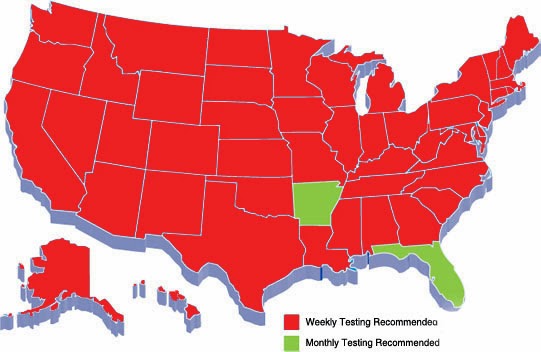 |
| Map of Sterilization Regulations for the US |
| Organizations | Biological Indicators | Chemical Indicators |
| CDC |
|
|
| OSHA |
|
|
| ADA |
|
|
For more information visit www.infectioncontrolproducts.com or call us at 800-366-0973

